5+ Fda Warning Letter 483
Its important to address each observation cited in the letter specifically and to respond in a way that is. Web FDA 483.

Fda Form 483 Response And Recovery Consulting Emergo By Ul
FDA Alerts Health Care Professionals not to Use Sterile Drug Products from Pharmakon Pharmaceuticals Inc Noblesville Indiana 04152016.

. Its purpose is to. This letter concerns your firms distribution of veterinary products for use in. If we look at the warning letters issued in the period from.
Web After issuing Form 483 FDA classifies Aurobindo plant as only needing voluntary action. Web Abstract Regular inspections are carried out to ensure system conformity by the Food and Drugs Regulatory Authority FDA of the United States one of the most. An FDA warning letter on the other hand is a more serious situation.
Web The FDA issued 121 warning letters to medical device manufacturers for violations of the Quality System Regulation in 2015. Does not constitute a final Agency determination. Web What is the purpose of an FDA Form 483.
After an inspection if the FDA investigator observes conditions that could be violations of FDA regulations they issue a. Web These observations are listed on an FDA Form 483 when in an investigators judgment the observed conditions or practices indicate that an FDA. Notifies management at the conclusion of an inspection of objectionable conditions.
Web Form FDA 483 2 Inspectional Observations is a form used by the FDA to document and communicate concerns discovered during these inspections. When responding to an FDA 483 clarity is key. The purpose of the FDA Form 483 is to notify the companys management of objectionable conditions.
By Joseph Keenan Dec 20 2023 953am. Web FDA 483 observations are listed on FDAs Inspectional Observations form when in the investigators judgment conditions or practices observed would indicate that any food. Web The Value of FDA 483s and Warning Letters.
Cites failure to adhere to federal regulations Summarizes issue provides examples Public. Web What is an FDA Warning Letter. Web WARNING LETTER.
Web An FDA Form 483 observation is a notice that highlights potential regulatory problems while a warning letter is an escalation of this notice. Web Critical Differences Between a Form FDA 483 and Warning Letters. You need to respond in.
Web Many medical device manufacturers receive FDA warning letters due to lack of preparation for the FDA inspection andor an inadequate response to an FDA. Investigator response Within 15 days and describes-Extent. Pharmaceutical Technology Pharmaceutical Technology July 2021 Issue Volume 45 Issue 7.
Form 483 List of Observations objectionable conditions MAY constitute. The most often-cited violations. Web A 483 is issued to a company at the end of an FDA inspection it documents any conditions that the inspector believes may violate FDA regulation.
Also referred to as Form. Web A search for the keyword stability also conducted on June 27 2023 returned a total of 260 results. Web FDA warning letter.
Web FDA Statement. A warning letter is usually issued for major compliance deficiencies. There are many publications and services that analyze the observations contained in Form FDA 483.
Web FDA 483 presented to Senior Management during inspection close-out.

Warning Letters And Notice Of Violation Letters To Pharmaceutical Companies Fda

Fda Warning Letters Und Formular 483

The Definitive Guide To Responding To Fda 483 Observations And Warning Letters
Fda Warning Letters Und Formular 483

Fda Warning Letter To Orthometrix Inc 2005 07 06 Circare
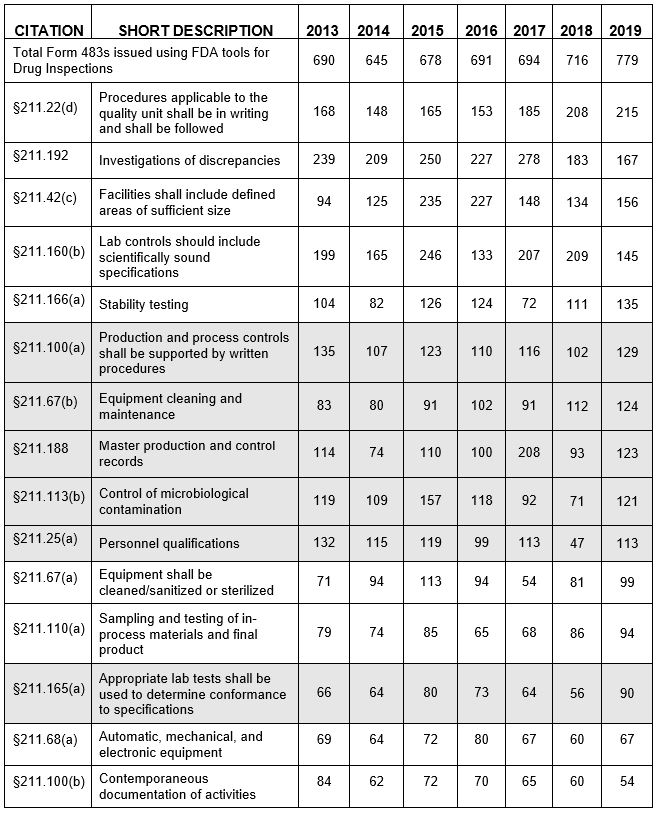
Fda Warning Letter Inspection Observation Trends Updated 2023

Understanding Basic Difference Between Form 483 Warning Letters Be Innov Tive

Assessment Of Usfda Warning Letters 483 S Related To Improper Handling Of Oos Results Veeprho

Warning Letters And Notice Of Violation Letters To Pharmaceutical Companies Fda

Why Firms Must Avoid Fda 483 And Warning Letters Pharmaguideline

Fda Form 483 And Warning Letters Pharmaguideline

Eleap All You Need To Know About Fda Form 483 And Warning Letter

Understanding The Implications Of An Fda 483 And Warning Letter

Successfully Responding To Fda Inspections 483s Warning Letters Ppt

Fda Form 483 Response And Recovery Consulting Emergo By Ul

Fda Warning Letter To Stuart A Harlin M D 2010 07 21 Circare

Fda Warning Letter Pdf Food And Drug Administration Pharmaceutical Sciences